An Atom's Atomic Mass Is Best Described As The Mass Of
An atom's atomic mass is best described as the mass of. This is not to be associated or mistaken for atomic weight. An atoms atomic mass is best described as the mass of. The protons it contains.
Celectrons in the outermost shell. Atomic mass on the other hand is the mass of the atom at rest and not in motion. A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom.
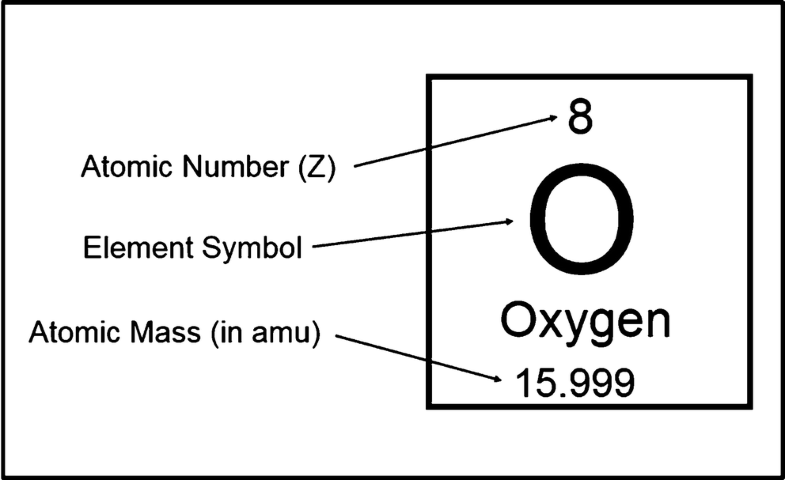
Atomic mass is the mass of an atom. In fact a unit outside the system an atomic mass unit is generally used. How do we account for the lower mass.
Bthe neutrons it contains. The answer to what is atomic mass is this. Atomic mass is additionally the total of protons and the sum of neutrons.
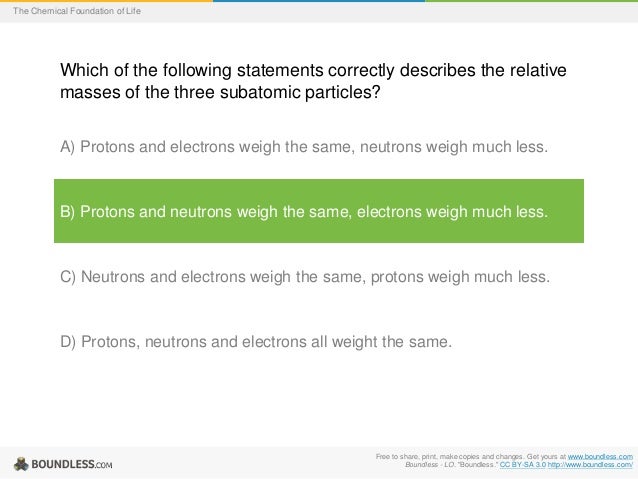
Protons and neutrons it contains. Electrons contribute so little mass that they arent counted. An atoms atomic mass is best described as the mass of Athe protons it contains.
Atomic mass is quantified via spectrometry. One of the fundamental properties of an atom is its mass. Therefore a hydrogen atom has a mass of approximately 167 10 -2 4 g.
Electrons in the outermost shell. The neutrons it contains.
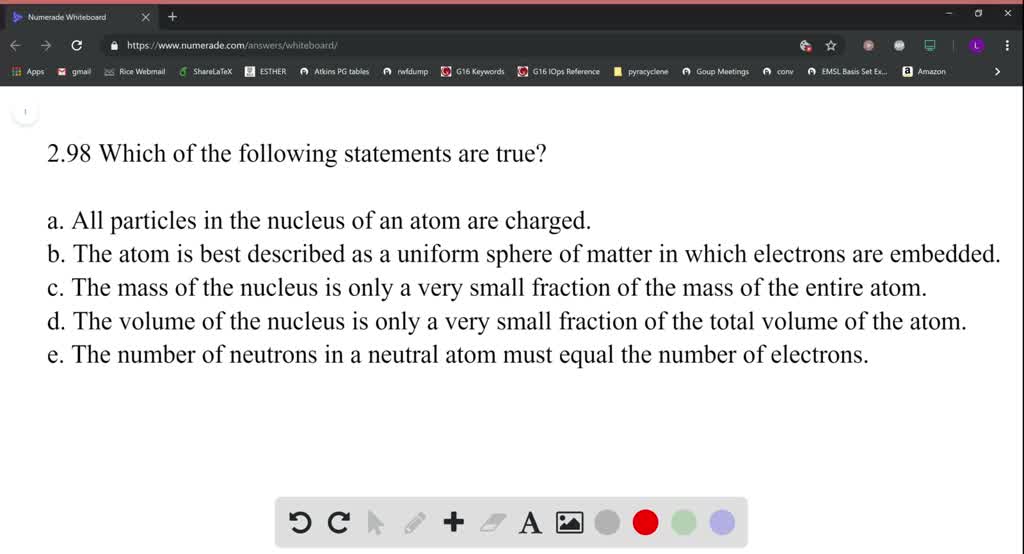
The absolute mass of an atom is an extremely small amount.
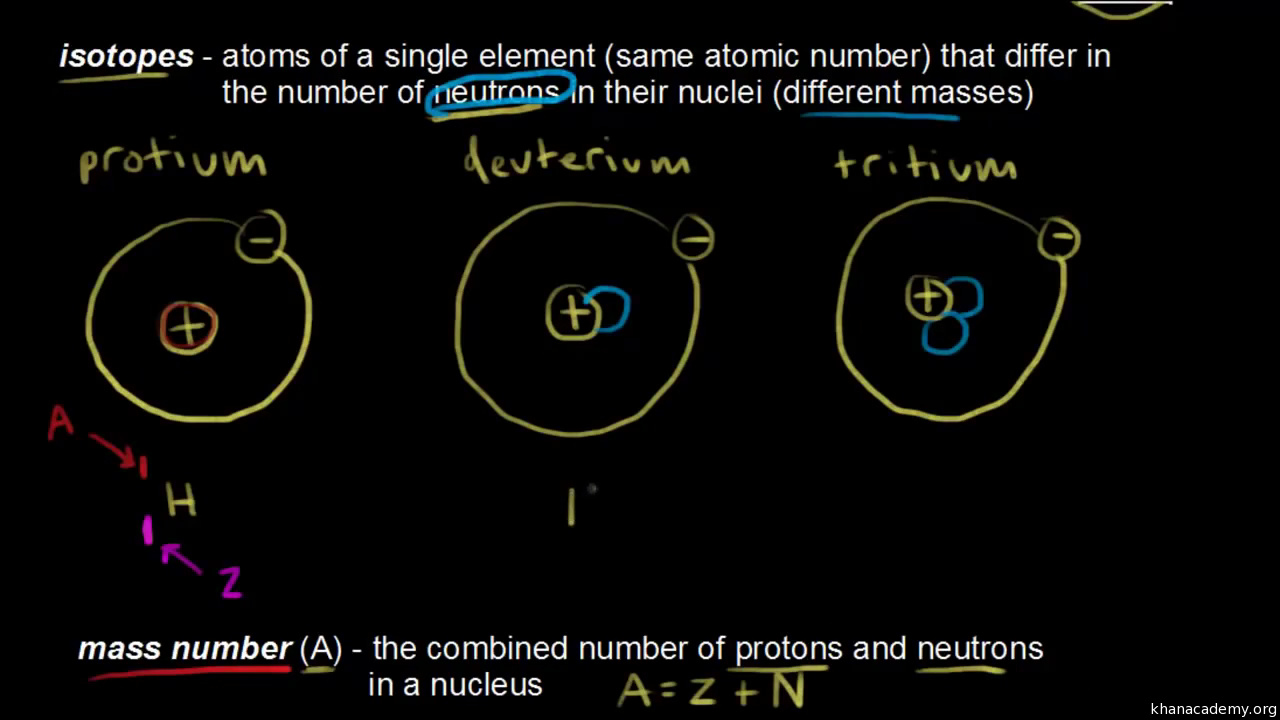
Atomic mass is additionally the total of protons and the sum of neutrons. An elements atomic mass is the weighted average of the masses of the isotopes of an element. An atoms atomic mass is best described as the mass of a protons it contains b from SCIENCE NA at Southwest Dekalb High School. Bthe neutrons it contains. Protons and electrons it contains. An elements atomic mass can be calculated provided the relative abundance of the elements naturally occurring isotopes and the masses of those isotopes are known. Atomic mass is the mass of an atom. This is less than the mean of the masses of 6 protons and 6 neutrons. Electrons contribute so little mass that they arent counted.
Full screen is unavailable. A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. Bthe neutrons it contains. Celectrons in the outermost shell. Atomic mass m a is the mass of an atom. Electrons in the outermost shell. Atomic mass is additionally the total of protons and the sum of neutrons.


:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)








/atomic-mass--58dc0d885f9b58468332c41b.jpg)


















Post a Comment for "An Atom's Atomic Mass Is Best Described As The Mass Of"